Polylactic Acid (PLA) Plastic
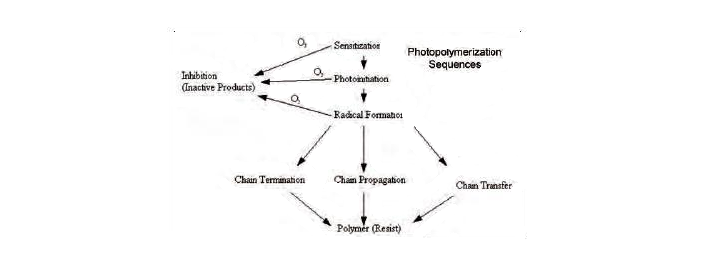
Photopolymerization is the polymerization of liquid by being exposed to light, typically ultraviolet (UV) light. Polymerization is the process of liquid turning to solid by building smaller molecules into larger molecules. Some common examples of products created by photopolymerization are plastic, such as Lego®, nylon, such as women’s stockings, and Teflon, used in cookware.
How Photopolymerization Works
Liquid exposed to UV light begins the process of photopolymerization with three main components: Binders or Oligomers, Monomers, and Photoinitiators.
Photoinitiators are molecules that split when exposed to UV light.
Monomers bind to other molecules to form a polymer and lower the viscosity (speed at which a liquid is moving).
Binder or Oligomers are a molecular complex consisting of a few monomers and are chain-like molecules that give the solid form it properties.
The molecules of binders and monomers combine to form chains and the chains bond to form a cross-linking pattern, which forms liquid into solid.
Photopolymerization in 3D Printing
Digital Light Processing (DLP) 3D printers use UV lights to harden, or create photopolymerization, of liquid polymer to create 3D printed objects. The liquid polymer is exposed to UV light which transposes the image of a 3D design onto the liquid, which hardens. The vat of liquid is drained layer by layer and repeatedly exposed to UV light until the object is complete.
Photopolymerization 3D Printers
The Objet series and the Dimension series by Stratasys are printers that use photopolymerization as well as several home-made models.
To learn more about the latest in 3D Printing, check out our 3D printing.